Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
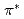 transitions in N
transitions in N O
OTravnikova, O.*; C olin, D.*; Bao, Z.*; B
olin, D.*; Bao, Z.*; B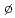 rve, K. J.*; Tanaka, Takahiro*; Hoshino, Masamitsu*; Kato, Hideki*; Tanaka, Hiroshi*; Harries, J.; Tamenori, Yusuke*; et al.
rve, K. J.*; Tanaka, Takahiro*; Hoshino, Masamitsu*; Kato, Hideki*; Tanaka, Hiroshi*; Harries, J.; Tamenori, Yusuke*; et al.
Journal of Electron Spectroscopy and Related Phenomena, 181(2-3), p.129 - 134, 2010/08
Times Cited Count:1 Percentile:7.38(Spectroscopy)In N O a detailed study of the vibrational distribution of the state reached after decay of core-to-
O a detailed study of the vibrational distribution of the state reached after decay of core-to-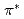 excitation of N terminal, N central and O 1s core levels is reported. We observe a change in the relative intensity of bending versus stretching modes while scanning the photon energy across all three resonances. While this effect is known to be due to the Renner-Teller splitting in the core-excited states, we could derive that the antisymmetric stretching is excited mainly in the decay of the N terminal 1s-to-
excitation of N terminal, N central and O 1s core levels is reported. We observe a change in the relative intensity of bending versus stretching modes while scanning the photon energy across all three resonances. While this effect is known to be due to the Renner-Teller splitting in the core-excited states, we could derive that the antisymmetric stretching is excited mainly in the decay of the N terminal 1s-to-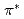 excitation. An explanation for such selectivity is provided in terms of interplay of vibrational structure on potential energy surfaces of different electronic states involved in the process.
excitation. An explanation for such selectivity is provided in terms of interplay of vibrational structure on potential energy surfaces of different electronic states involved in the process.
Hosaka, Koichi; Itakura, Ryuji; Yokoyama, Keiichi; Yamanouchi, Kaoru*; Yokoyama, Atsushi
no journal, ,
Fujii, Kentaro; Yokoya, Akinari
no journal, ,
To in-vestigate the mechanism of the molecular change in DNA irradiated with the soft X-rays, we have measured the spectra of the near edge X-ray absorption fine structure (NEXAFS) of DNA caused by exposure to monochromatic soft X-rays. We used a calf thymus DNA and oligonucleotide thin films as samples and observed N K-shell and O K-shell NEXAFS spectral changes. The typical monochromatic soft X-ray energies used for the irradiation (380, 435, 560, and 760 eV) were obtained from SPring-8, BL23SU. The observed spectra showed that initiation of the new products by the irradiation in the irradiated sample and the dissociation of molecular structure of the DNA by the irradiation. By comparison of these NEXAFS spectral changes with the yields of base lesions and strand breaks as final products, we will discuss the molecular structure of DNA damage site and the site selectivity of damage induction in DNA by soft X-rays.
Shimoyama, Iwao; Baba, Yuji; Sekiguchi, Tetsuhiro; Uddin, M. N.*; Nagano, Masamitsu*
no journal, ,
Graphite-like boron carbonitride (B-C-N) have been expected to control a wide variety of electronic properties. However, complicated chemical bonding make it difficult to analyze the atomic arrangement between B, C and N has not been well clarified. We used NEXAFS spectroscopy for characterization of local structures of B-C-N thin films. In both B and N K-edge NEXAFS spectra, some  *peaks of B-C-N compounds and the
*peaks of B-C-N compounds and the  * peaks of hexagonal boron nitride (h-BN) appeared with graphite-like polarization dependence. We calculated partial density of states (PDOS) of B and N sites in some models clusters of graphite-like B-C-N by ab initio molecular orbital calculation. Based on the comparison of theoretical results with NEXAFS spectra, we propose a rule that B, C, and N atoms are arranged not to inhibit interatomic polarization between B and N sites in graphite-like structures.
* peaks of hexagonal boron nitride (h-BN) appeared with graphite-like polarization dependence. We calculated partial density of states (PDOS) of B and N sites in some models clusters of graphite-like B-C-N by ab initio molecular orbital calculation. Based on the comparison of theoretical results with NEXAFS spectra, we propose a rule that B, C, and N atoms are arranged not to inhibit interatomic polarization between B and N sites in graphite-like structures.
Kosuwattage, K.; Shimoyama, Iwao; Baba, Yuji; Sekiguchi, Tetsuhiro; Nakagawa, Kazumichi*
no journal, ,
B-C-N nanomaterials have attracted attention for hydrogen storage. We investigated adsorption and desorption of hydrogen on hexagonal boron nitride (h-BN) and graphite thin films which were chosen as basic systems of the B-C-N nanomaterials using XPS. Epitaxial thin films of h-BN and graphite were deposited on Ni(111) surfaces by CVD method, and were exposed to atomic hydrogen. B 1s and N 1s spectra of h-BN thin films and C 1s spectra of graphite thin films showed peak shifting and peak broadening after hydrogen treatments. We quantitatively compared hydrogenation for h-BN and graphite thin films through peak fitting analysis, and obtained preliminary result that h-BN may have larger degree of hydrogenation larger than graphite. We also studied the hydrogen desorption properties and found that the initiation temperature of desorption for h-BN is similar to the case of graphite. These results suggest that BN-based material can be a potential material for hydrogen storage.
 molecular beam
molecular beamHashinoguchi, Michihiro*; Okada, Michio*; Yoshigoe, Akitaka; Teraoka, Yuden
no journal, ,
We have studied the oxidation processes on Cu(100), Cu(111) and Cu(110) surfaces at room temperature using hyperthermal O molecular beam. A collision-induced absorption mechanism was proposed for the oxidation process of Cu into Cu
molecular beam. A collision-induced absorption mechanism was proposed for the oxidation process of Cu into Cu O on Cu(100) and Cu(111). On the other hand, an additional mechanism involving mobile Cu adatoms was suggested for Cu(110). Thus, surface temperature is expected to be a key factor of Cu
O on Cu(100) and Cu(111). On the other hand, an additional mechanism involving mobile Cu adatoms was suggested for Cu(110). Thus, surface temperature is expected to be a key factor of Cu O growth on Cu(110). Here, we present surface temperature dependence of oxidation processes on Cu(110) using molecular beams and X-ray photoemission spectroscopy in conjunction with synchrotron radiation. All experiments were performed with the surface reaction analysis apparatus constructed at BL23SU in SPring-8. We observed the efficient oxide formation on Cu(110) at 473 K in comparison with 300 K for the 2.2 eV incidence.
O growth on Cu(110). Here, we present surface temperature dependence of oxidation processes on Cu(110) using molecular beams and X-ray photoemission spectroscopy in conjunction with synchrotron radiation. All experiments were performed with the surface reaction analysis apparatus constructed at BL23SU in SPring-8. We observed the efficient oxide formation on Cu(110) at 473 K in comparison with 300 K for the 2.2 eV incidence.
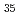 V
V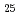 Cr
Cr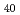 by photoemission spectroscopy
by photoemission spectroscopyHaruyama, Yuichi*; Teraoka, Yuden; Matsui, Shinji*
no journal, ,
In this study, we have investigated the chemical composition and the electronic structure near surface region in the ternary transition metal alloy Ti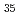 V
V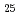 Cr
Cr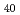 by means of photoemission spectroscopy. The photoemission spectra in Ti
by means of photoemission spectroscopy. The photoemission spectra in Ti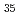 V
V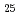 Cr
Cr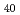 were measured as a function of annealing temperature and photon energy. With increasing annealing temperature, the intensity originating from Ti increased. This indicates that the redistribution of the constituent transition metals was caused by the annealing. From the photon energy dependence of the energy distribution curves and constant initial state spectra in the 3p-3d resonant photoemission region of each transition metal, the orbital character of the observed features in the valence band region was clarified.
were measured as a function of annealing temperature and photon energy. With increasing annealing temperature, the intensity originating from Ti increased. This indicates that the redistribution of the constituent transition metals was caused by the annealing. From the photon energy dependence of the energy distribution curves and constant initial state spectra in the 3p-3d resonant photoemission region of each transition metal, the orbital character of the observed features in the valence band region was clarified.
 molecular beams
molecular beamsTeraoka, Yuden; Kawakami, Yasunori; Yoshigoe, Akitaka; Harries, J.; Hiraya, Atsunari*
no journal, ,
Chemical bonding states of oxygen, adsorbed on Ni(111) via supersonic O beams have been analyzed by low energy electron diffraction and synchrotron radiation X-ray photoemission spectroscopy. The oxygen-adsorbed structure in the early stages of O
beams have been analyzed by low energy electron diffraction and synchrotron radiation X-ray photoemission spectroscopy. The oxygen-adsorbed structure in the early stages of O irradiation was assigned to be an three-folded near-fcc site. The oxide layer consisted of peroxide, suboxide, and NiO components depending on O
irradiation was assigned to be an three-folded near-fcc site. The oxide layer consisted of peroxide, suboxide, and NiO components depending on O incident energy and dose. In the 0.6 eV case, the three-folded near-fcc site changed finally to the NiO through suboxide and peroxide. The oxide layer was NiO(111) regardless of kinetic energy. With increasing kinetic energy, NiO formed in the lower O
incident energy and dose. In the 0.6 eV case, the three-folded near-fcc site changed finally to the NiO through suboxide and peroxide. The oxide layer was NiO(111) regardless of kinetic energy. With increasing kinetic energy, NiO formed in the lower O dose and the three-folded near-fcc sites decreased in the earlier stages. Especially, the kinetic energy of 2.3 eV was effective to form NiO in the extreme early stages. This implies that the activated adsorption occurs in the oxidation of Ni(111) by energetic O
dose and the three-folded near-fcc sites decreased in the earlier stages. Especially, the kinetic energy of 2.3 eV was effective to form NiO in the extreme early stages. This implies that the activated adsorption occurs in the oxidation of Ni(111) by energetic O molecules.
molecules.